Mechanochemically induced recycling of lithium-ion batteries

Used lithium-ion batteries have a high resource value considering the scarcity of raw materials. They contain valuable materials that could be reused with systematic recycling. The increasing use of these batteries requires economical and ecologically sound recycling technologies.
State of the art
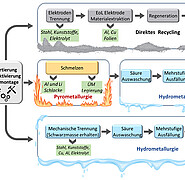
The recovery of lithium is currently still expensive and not very profitable. One usal process is pyrometallurgy, in which dismantled and shredded batteries are melted at over 1000 degrees Celsius in the absence of oxygen. Valuable metals are then separated in the resulting alloys using a multi-stage leaching process. Lithium and graphite are partially lost with the non-recyclable slag and flue gas. In hydrometallurgy, battery components are also separated by means of strong acids and bases. What both technologies have in common is that they leave behind harmful by-products and use corrosive reagents.
Technology
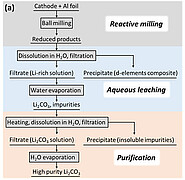
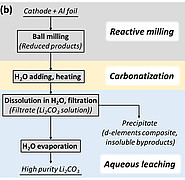
Researchers at the Institute for Applied Materials (IAM-ESS) have developed a more sustainable approach to recovering lithium and transition metals from batteries. High-energy ball milling at room temperature is used to mechanochemically initiate a chemical reaction between cathode materials and aluminum as a reducing agent. The reduction reaction generates reaction products containing water-soluble lithium and aluminum, which are afterwards washed out. After evaporation of the water-soluble fraction, the lithium carbonate is separated from the aluminum oxide by heating and washing again. Transition metals such as cobalt and nickel are present as solid, finely powdered metal composites and can be dissolved in dilute mineral acids at room temperature without the need for additional reducing agents. In a modified process, predominantly water-soluble lithium carbonate can already be generated during the first wash by adding a carbonate source.
Advantages
The process developed is therefore not only environmentally friendly, economical and energy-efficient, but can also be used universally for popular cathode materials (NMC, LCO, LMO, LFP). The simple process enables the recovery of valuable battery components and achieves yields of up to 70 percent for lithium and over 90 percent for transition metals.
Options for companies
As the process was initially developed on a laboratory scale with quantities of up to 10 grams, KIT is actively looking for partners for further development and scaling up.
Images close open
Your contact person for this offer

Innovation Manager New Materials and Health Technologies Karlsruhe Institute of Technology (KIT)
Innovation and Relations Management (IRM) Phone: +49 721 608-26107
Email: jan-niklas.bloetz@kit.edu